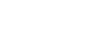| 证券代码 | AIMT.O |
| 证券名称 | Aimmune Therapeutics Inc |
| 证券类型 | 美股 |
| 上市场所 | 纳斯达克交易所 |
| 上市板块 | 主板 |
| 发行方式 | 公开发售 |
| 首发上市日 | 2015-08-06 |
| 首发价格(元) | 16 USD |
| 首发数量(股) | 10000000 |
| 首发募资额(元) | 160,000,000.00 USD |
| 首发主承销商 | Credit Suisse Securities (USA) LLC,Piper Jaffray & Co,BofA Merri |
| 货币单位 | USD |
| 公司名称 | Aimmune Therapeutics, Inc. |
| 注册地址 | 美国特拉华州 |
| 办公地址 | 8000 Marina Blvd Suite 300, Brisbane, California, USA |
| 成立日期 | 2011-06-24 |
| 董事会主席 | Mark D. McDade |
| 公司属地 | United States 美国 |
| 公司网址 | www.aimmune.com |
| 电话 | +1 (650) 614-5220 |
| 传真 | +1 (650) 616-0075 |
| 公司简介 | Aimmune Therapeutics, Inc., is a biopharmaceutical company developing treatments for life-threatening food allergies. The company’s Characterized Oral Desensitization ImmunoTherapy approach is intended to provide meaningful levels of protection against allergic reactions resulting from accidental exposure to food allergens by desensitizing patients with defined, precise amounts of key allergens. Aimmune’s first investigational biologic product using Characterized Oral Desensitization ImmunoTherapy, AR101 for the treatment of peanut allergy, has received the FDA’s Breakthrough Therapy Designation for the desensitization of peanut-allergic patients 4-17 years of age. Aimmune plans to submit regulatory filings for marketing approval of AR101 in the United States and Europe based on data from the pivotal Phase 3 PALISADE clinical trial of AR101, which in 4-17 year-old subjects met all its primary and secondary endpoints, and additional ongoing and completed AR101 clinical trials. |